
蔡 晃植
(さい・こうしょく)
Fang-Sik Che
略歴
- 朝鮮大学理学部卒業
- 理化学研究所基礎科学特別研究員、奈良先端科学技術大学院大学助手を経て本学へ
植物分子環境生理学研究室

卒業研究テーマ例
- 植物による病原菌分子パターンやエフェクター分子の認識と植物免疫反応の誘導機構
- 伝承野菜の遺伝的解析と自家不和合性解析による優良系統の育種
- 植物の環境認識機構の解明と代謝経路の分子制御による有用植物の作成
植物における病原菌認識の分子機構解析
植物免疫反応の誘導機構解析
植物における環境認識機構の分子機構解析
植物工場を用いた高機能野菜作出
また、植物の免疫反応の一つである過敏感細胞死誘導の機構についても研究を行い、イネの過敏感細胞死誘導においてOsNAC4という転写因子が細胞死誘導の分子スイッチとして機能していることを明らかにした。病原菌認識情報によって発現誘導されたOsNAC4はリン酸化されることによって細胞質から核に移行し、同じサブグループに存在するOsNAC3と結合し、OsHSP90やヌクレアーゼであるIRENをコードする遺伝子の発現を誘導する。OsHSP90は小胞体に移行し、細胞膜透過性の喪失を引き起こし、IRENは核に移行し、核DNAの断片化を行うことで植物の過敏感細胞死を引き起こしていることを明らかにした。
現在では、植物が低温を感知して凍結温度でも耐えられるように自己を変化させる機構についても研究を行っている。これまで、低温処理無しで凍結耐性能力を獲得したシロイヌナズナ変異株を複数選抜し、これらの原因遺伝子について解析を行うことで、植物の低温感知能力と凍結耐性能力について知見を得ている。今後は、上記のような基礎研究結果を基に、病原菌に対して抵抗性を示す耐病性植物の作出や低温耐性植物の作出、植物に耐病性を付加する化学物質の開発、植物工場での機能性植物を通した肥満防止薬や血糖値低下薬などの開発にも携わっていきたい。
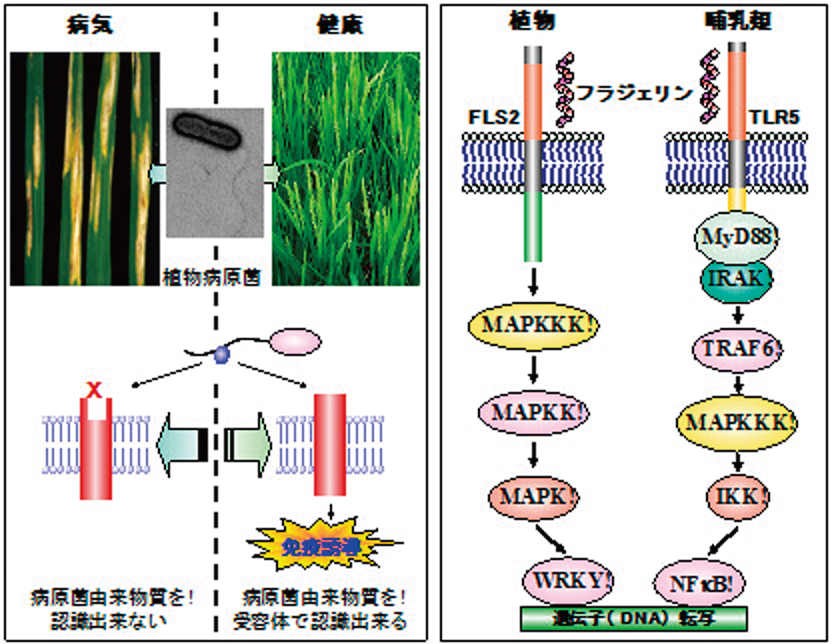
(図1 左:植物における病原細菌認識と免疫反応誘導の模式図。植物が病原菌を感知して植物免疫反応を誘導した場合、病気は起きないが、植物が病原菌を認識出来ない場合、植物は発病する。
右:植物と哺乳類に存在する細菌の鞭毛タンパク質フラジェリンの認識システムの模式図。植物と哺乳類では非常に類似したシステムでフラジェリンを認識し免疫を誘導していることがわかる。)
- 研究の応用領域
- 農薬、耐病性、植物成長調節剤、植物新品種作成、植物工場
- 産官学連携で求めるパートナー
- 農薬開発企業、植物種子関係企業、植物生理研究者、組み換え植物関係企業
During development, plants are continuously confronted with diverse pathogens. However, plants are resistant to most microbes and rely entirely on plant immune responses for their defense. Plants have evolved a multi-layered defense system that can be activated upon pathogen invasion. Flagellin, a main component of the bacterial flagellum, from the rice avirulent N1141 strain of gram-negative phytopathogenic bacterium, Acidovorax avenae, induces plant immune responses including H2O2 generation, while flagellin from the rice virulent K1 strain of A. avenae does not induce these immune responses. To clarify the molecular mechanism that leads to these differing responses between the K1 and N1141 flagellins, recombinant K1 and N1141 flagellins were generated using an Escherichia coli expression system. When cultured rice cells were treated with recombinant K1 or N1141 flagellin, both flagellins equally induced H2O2 generation, suggesting that post-translational modifications of the flagellins are involved in the specific induction of immune responses. Mass spectrometry analyses using glycosyltransferasedeficient mutants showed that 1,600 Da and 2,150 Da glycans were present on the flagellins from N1141 and K1, respectively. A deglycosylated K1 flagellin induced immune responses in the same manner as N1141 flagellin. Site-directed mutagenesis revealed that glycans were attached to four amino acid residues (178Ser, 183Ser, 212Ser and 351Thr) in K1 flagellin. Among mutant K1 flagellins in which each glycan-attached amino acid residue was changed to alanine, 178Ser/Ala and 183Ser/Ala K1 flagellin induced a strong immune response in cultured rice cells, indicating that the glycans at 178Ser and 183Ser in K1 flagellin prevent epitope recognition in rice.
We next examined additional factors, distinct from flagellin, that act as inducers of immune responses in rice. Cell extracts isolated from flagellin-deficient N1141 (Df la1141) still induced PTI responses, suggesting that Df la1141 possesses an additional PAMP distinct from flagellin. Here, we show that elongation factor Tu (EF-Tu), one of the most abundant bacterial proteins, act as a PAMP in rice and causes several PTI responses. In Brassicaceae species, EF-Tu and an N-acetylated peptide comprising the first 18 amino acids of the N-terminus, termed elf18, are fully active as inducers of PTI responses. By contrast, elf18 did not cause any immune responses in rice, whereas an EF-Tu middle region comprising Lys176 to Gly225, termed EFa50, is fully active as a PAMP in rice. In the leaves of rice plants, EF-Tu induced H2O2 generation and callose deposition, and also triggered resistance to co-infection with pathogenic bacteria. Taken together, these data demonstrate that rice recognizes EFa50, which is distinct from elf18, and that this epitope induces PTI responses.
To clarify the induction mechanism of Hypersensitive cell death (HR), we identified several genes that are upregulated during HR cell death using PCR subtraction analysis and microarray analysis. Among the identified genes, the OsNAC4 gene encoding a plant-specific transcription factor was identified in both analyses. Overexpression of OsNAC4 leads to HR cell death accompanied by the loss of plasma membrane integrity, nuclear DNA fragmentation and typical morphological changes. In OsNAC4 knock-down lines, HR cell death is markedly decreased in response to avirulent bacterial strains. After induction by an avirulent pathogen recognition signal, OsNAC4 is translocated into the nucleus in a phosphorylation-dependent manner. To understand the molecular mechanisms by which OsNAC4 mediates cell death, we compared global gene expression in the OsNAC4-RNAi cell line with a control line using a 22,000 elements oligonucleotide microarray. A microarray analysis demonstrated that 139 genes including OsHSP90 and IREN, encoding a Ca2+-dependent nuclease, were expressed differently between the OsNAC4-RNAi and control lines during HR cell death. When OsHSP90 was transiently expressed in rice cells, loss of plasma membrane integrity was rapidly induced, while no nuclear DNA fragmentation was observed. We next cloned the full-length IREN cDNA into a vector under the control of the ubiquitin promoter, and introduced this construct into rice cells. After TUNEL staining, all of the IREN-expressing cultured rice cells exhibited fluorescein-derived bright-green fluorescence of their nuclei, suggesting that IREN overexpression causes rapid DNA fragmentation in nuclei. While no cell death induction was detected when IREN was transiently expressed in rice protoplasts. Overall, our results indicate that two important events occurring during HR cell death are regulated by independent pathways.
Kondo, M., Hirai, H., Furukawa, T., Yoshida, Y., Suzuki, A. and Che, F. S. (2017) Frameshift mutation confers function as virulence factor to leucine-rich repeat protein from Acidovorax avenae. Frontiers in Plant Science, 7, 1-13.
Ootsubo, Y., Hibino, T., Wakazono T., Mukai Y, and Che, F. S. (2016) IREN, a novel EF-hand motif-containing nuclease, functions in the degradation of nuclear DNA during the hypersensitive response cell death in rice. Biosci. Biotech. Biochem., 80, 748-760.
Katsuragi, Y., Takai, R., Furukawa, T., Hirai, H., Morimoto, T., Katayama, T., Murakami, T. and Che, F. S. (2015) CD2–1, the C-terminal region of flagellin, modulates the induction of immune responses in rice. Mol. Plant Microb. Interact., 28, 648-658.
Kaneda, T., Taga, Y., Takai, R., Iwano, M., Matsui, H., Takayama, S., Isogai, A., Che, F. S. (2009) The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J., 28, 926-936.
Murase K., Shiba H., Iwano M., Che F. S., Watanabe M., Isogai A., Takayama S. (2004) A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science, 303, 1516-1519.






