メディカルバイオサイエンス学科
 中村 卓(なかむら・たかし)
中村 卓(なかむら・たかし)
Takashi Nakamura
専門分野/生物有機化学、タンパク質工学
研究キーワード/酵素、コンピュータシミュレーション、有害物質分解、有用物質生産
職位:准教授
学位:博士(工学)(京都大学)
- 京都大学大学院工学研究科博士課程修了
- 独立行政法人理化学研究所・国立環境研究所研究員、山田科学振興財団長期派遣援助研究員(チェコ・マサリク大学)を経て本学へ
研究テーマ
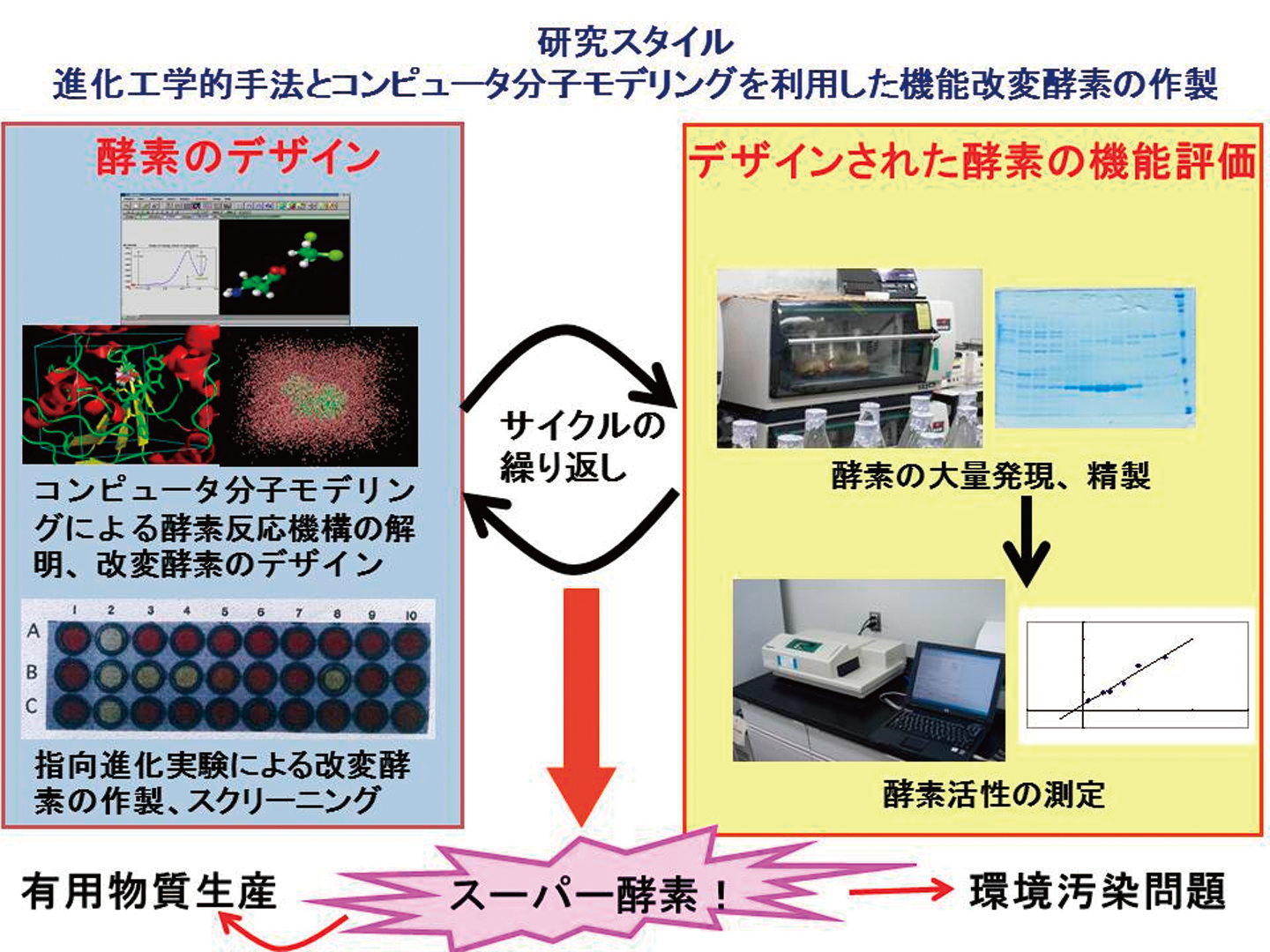
コンピュータ分子モデリングによる分子レベルでの酵素反応機構解析とその知見を利用した改変酵素のデザインと作製、あるいは進化工学的手法による改変酵素の作製を通して環境汚染物質の分解や産業的に有用な物質の生産に貢献したいと考えている。
現在は、以下の3つの酵素について研究を行っている。
(1) 残留性農薬やダイオキシン類などによく見られる、有機ハロゲン化合物の一種であるハロ酸を分解する、ハロ酸脱ハロゲン化酵素L-DEX YL
(2) 医薬品やその前駆物質として極めて有用性の高い非天然アミノ酸を合成することができる、超好熱性古細菌由来の耐熱性システイン合成酵素OPSS
(3)効率のよいシステイン発酵生産を可能にする大腸菌由来システイン合成酵素OASSL-DEX YL は有害物質分解へ、OPSSとOASS は有用物質生産への応用を目指している。
| 研究の応用領域 | 産官学連携で求めるパートナー |
|---|---|
| 環境汚染物質の検出(バイオセンサー)、環境汚染物質浄化槽への利用(バイオリアクター)、光学活性物質などの有用物質生産 | 独立行政法人研究機関、国内外の大学や研究機関、バイオ系企業、化学系企業、ベンチャー企業 |
Topics of research
Enzymes are molecular machines that catalyze chemical reactions in living organisms. It is of highly scientif ic interest and practical need to construct enzymes with new catalytic properties and enhanced stability. Tailor-made biocatalysts can be created from wild-type enzymes by protein engineering using computer-aided molecular modeling and site-directed mutagenesis (rational design) or by directed evolution techniques (random mutagenesis).
Rational design usually requires both the availability of the structure of the enzyme and knowledge about the relationships between sequence, structure, mechanism, and function. It is therefore very information-intensive. Using molecular modeling, it has been possible to predict how to increase the selectivity, activity and stability of enzymes.
In contrast, directed evolution involves either a random mutagenesis of the gene encoding the catalyst (e.g. errorprone PCR) or recombination of gene fragments (e.g. DNA shuf f ling). Libraries thus created are usually assayed using high-throughput technologies to identify improved variants. Recently, combination of computational protein design with directed evolution is often used for creating new enzymes and successful results are obtained in some cases.
The object of my research is to design highly functional enzymes by protein engineering techniques as mentioned above. There are two kinds of enzymes I would like to design. The f irst enzyme is L-2-haloacid dehalogenase (L-DEX). This enzyme catalyzes the hydrolytic dehalogenation of L-2-haloalkanoic acids to produce the corresponding D-2- hydroxyalkanoic acids. Our goal to the design of L-DEX is to produce the enzyme which can detoxify toxic organichalogenated compounds.
The second enzyme is O-phosphoserine sulfhydrylase (OPSS). OPSS is found in a hyperthermophilic aerobic archaeon, Aeropyrum permnix K1. It is stable at high temperatures as high as 80℃ and produces cysteine more ef f iciently than other cysteine synthases. It may have potential applications for the ef f icient production of unnatural amino acids, which are applicable to building blocks for the synthesis of pharmaceuticals and agrochemicals. Thus, we are now investigating such a possibility by activity measurement, X-ray crystallography, and molecular modeling.
The third enzyme is O-acetylserine sulfhydrylase (OASS) from Escherichia coli. OASS can produce cysteine and unnatural amino acids as OPSS. We are now trying to construct OASS mutants applicable to the method for the production of L-cysteine by fermentation.
主な業績論文等
- Nakamura, T., Kunimoto, K., Yuki, T., Ishikawa, K. (2017) Unnatural Amino Acid Synthesis by Thermostable O-Phospho-L-Serine Sulfhydrylase from Hyperthermophilic Archaeon Aeropyrum pernix K1. Chem. Lett. 46, 1789-1792.
- Takeda, E., Kunimoto, K., Kawai, Y., Kataoka, M., Ishikawa, K., Nakamura, T. (2016) Role of F225 in O-phosphoserine sulfhydrylase from Aeropyrum pernix K1. Extremophiles 20 733–745.
- Nakamura, T., Asai, S., Nakata, K., Kunimoto, K., Oguri, M., Ishikawa, K. (2015) Thermostability and Reactivity in Organic Solvent of O-Phospho-L-Serine Sulfhydrylase from Hyperthermophilic Archaeon Aeropyrum pernix K1. Biosci. Biotechnol. Biochem. 79, 1280-1286.
- Kondo, H. Hujimoto, J. K., Tanaka, S., Deki, H., Nakamura, T. (2015) Theoretical prediction and experimental verification on enantioselectivity of haloacid dehalogenase L-DEX YL with chloropropionate. Chem. Phys. Lett. 623, 101-107.
- Kondo, H., Nakamura, T., Tanaka, S. (2014) A significant role of Arg41 residue in the enzymatic reaction of haloacid dehalogenase L-DEX YL studied by QM/MM method. J.Mol. Cat. B: Enzymatic. 110, 23-31.
- Nakamura, T., Kawai, Y., Kunimoto, K., Iwasaki, Y., Nishii K., Kataoka, M., Ishikawa, K. (2012) Structural analysis of the substrate recognition mechanism in O-phosphoserine sulfhydrylase from the hyperthermophilic archaeon Aeropyrum pernix K1. J. Mol. Biol. 422, 33-44.






