
小宮 徹
(こみや・とおる)
Tohru Komiya
略歴
- 九州大学大学院医学系研究院博士課程修了
- 九州大学大学院医学系研究院助手を経て本学へ
細胞工学研究室
卒業研究テーマ例
- 酸化ストレスによって誘導される細胞死のメカニズム
- 細胞内エネルギー代謝の調節と細胞死の関係についての解析
- ミトコンドリアへのタンパク輸送のメカニズム
キーワード:ミトコンドリア、細胞内タンパク輸送、細胞死、酸化還元システム
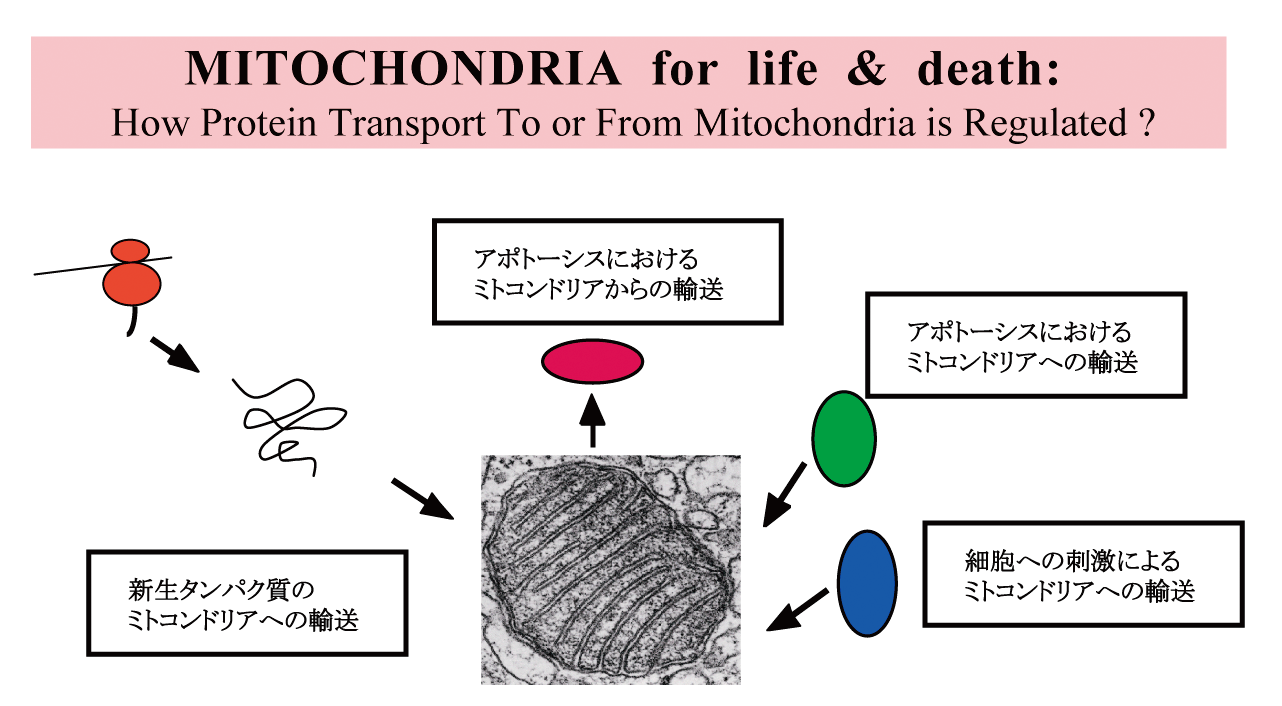
ミトコンドリアのタンパク質の局在化の分子機構
酸化ストレス誘導性細胞死とミトコンドリアの関係
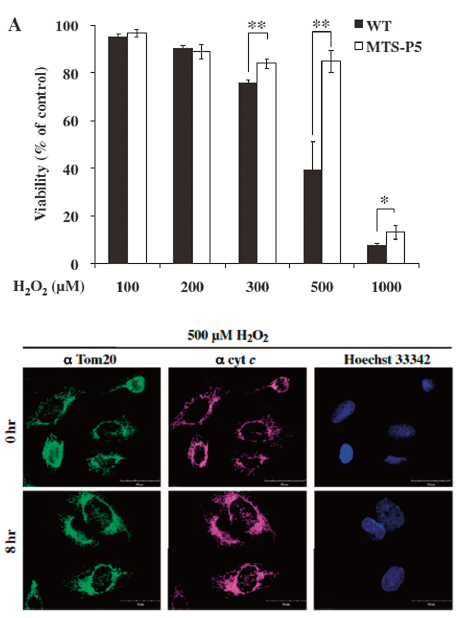
(下図)ミトコンドリア局在型P5安定発現細胞(MTS-P5)におけるcytochrome c放出の抑制。H2O2処理8時間後の免疫染色の結果を示した。MTS-P5ではcytochrome cの放出が抑制されている(参考文献2より抜粋)。
- 研究の応用領域
- 高次生命活動におけるオルガネラの機能解析、オルガネラの機能の異常による病因の解析
- 産官学連携で求めるパートナー
- 国、地方自治体、国立研究所、医療研究機関、バイオ系ベンチャー企業
Mitochondria, one of the organelles in the eukaryotic cells, have been extensively studied. The major mitochondrial function is to generate ATP, the energy current molecule, through the tricarboxylic acid (TCA) cycle and the oxidative phosphorylation (OXPHOS). Mitochondria also carry out several important metabolic reactions such as lipid β-oxidation, urea cycle, heme biosynthesis, iron-sulfer cluster biosynthesis, to name
Our interests are divided into two main themes as follows:
How a dually-localized protein is sorted between mitochondria and the endoplasmic reticulum?
Most mitochondrial proteins are nuclear-coded, synthesized on cytosolic free ribosomes as precursor with mitochondrial targeting signal (MTS), then transported to mitochondria. While extensive studies are made, some proteins are reported to be transported to dual destinations: mitochondria and the other compartment(s) in the cell. Among these we have clarified that P5, a member of protein disulfide isomerase family, is transported not only to the endoplasmic reticulum, but also to mitochondria. Now we study on how P5 is sorted to these two organells and on the putative mitochondrial targeting signal(s) in P5.
How are linked between the mitochondrial dysfunction(s) and oxidative stress-induced cell death?
Defects in mitochondrial function cause not only a reduced energy production, but also an increased generation of reactive oxygen species (ROS), damage on mitochondrial morphology (in particular cristae), and if such defects are severe, damaged cells are destined to cell death. On the other hand, mitochondrial dysfunction has been suggested to be associated with numerous diseases, including cancer, diabetes, and neurodegenerative disease (Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, Amyotrophic Lateral Sclerosis, etc.). Despite its significance and extensive studies, in many cases, the relationships among oxidative stress-induced cell death, mitochondrial dysfunction, and causative effect(s) on diseases described above, are not well clarified so far.
Recently we have found that mitochondrial P5 suppresses the oxidative stress-induced cell death. Now we study the molecular mechanism(s) underlying the cell death suppression, and the relationships between intracelluar metabolism and cell death mechanisms.
Honjo, Y., Horibe, T., Torisawa, A., Ito, H., Nakanishi, A., Mori, H., Komiya, T., Takahashi, R. and Kawakami, K. (2014) Protein Disulfide Isomerase P5- Immunopositive Inclusions in Patients with Alzheimer’s Disease., J. Alz. Dis., 38, 601-609.
Shitara, Y., Tonohora, Y., Goto, T., Yamada, Y., Miki, T., Makino, H., Miwa, M. and Komiya, T. (2012)Mitochondrial P5, a member of protein disulf ide isomerase family, suppresses oxidative stress-induced cell death., J. Biochem., 152, 73-85.
Kamemura, K., Ogawa, M., Ohkubo, S., Ohtsuka, Y., Shitara, Y., Komiya, T., Maeda, S., Ito, A. and Yoshida, M. (2012) Depression of mitochondrial metabolism by downregulation of cytoplasmic deacetylase, HDAC6., FEBS Lett., 586. 1379-1383.
Kimura, T., Horibe, T., Sakamoto, C., Shitara, Y., Fujiwara, F., Komiya, T., Yamamoto, A., Hayano, T., Takahashi, N. and Kikuchi, M. (2008) Evidence for Mitochondrial Localization of P5, a Member of the Protein Disulf ide Isomerase Family. J. Biochem., 144, 187-196.
Suzuki, H., Okazawa, Y., Komiya, T., Saeki, K., Mekada, E., Kitada, S., Ito, A. and Mihara, K. (2000) Characterization of rat TOM40, a Central Component of the Preprotein Translocase of the Mitochondrial Outer Membrane. J. Biol. Chem., 275, 37930-37936.






