
岩本(木原) 昌子
(いわもと(きはら)・あつこ)
Atsuko Iwamoto-Kihara
略歴
- 大阪大学大学院工学研究科博士課程修了
- 大阪大学産業科学研究所教務職員、東京大学大学院総合文化研究科助手を経て本学へ
細胞機能学研究室
卒業研究テーマ例
- ミュータンス菌の耐酸性機構;プロトン排出ポンプ酵素の仕組み
- ATP合成酵素の遺伝子への変異導入
- F型ATPアーゼの回転型モーター酵素としての性質の解明
ナノモーターであるATP 合成酵素の回転制御
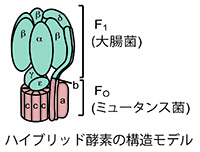
ミュータンス菌F 型プロトンATPaseの性状解析
- 研究の応用領域
- エネルギー変換デバイス
虫歯予防薬などの開発 - 産官学連携で求めるパートナー
- 生命科学分野あるいは工学分野の研究者・研究機関
1

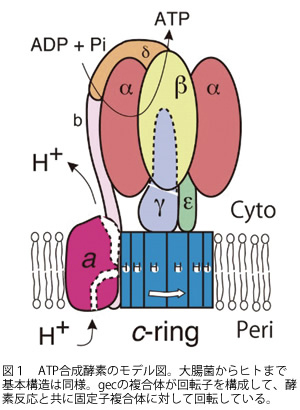
ATP (adenosine 5′-triphosphate) is an “energy currency” of the cells from bacterium through human. ATP is synthesized utilizing energy included in food molecules or sun light, and is hydrolyzed in energy consuming cellular events, such as muscular contraction, active transport of ions and nutrient, anabolic reactions, etc. ATP synthase is ubiquitously distributed in biological membranes, in which proton motive force (electrochemical potential of H+) was converted to the chemical energy of ATP. When protons were transported through the H+ pathway of the enzyme, ATP is synthesized on the catalytic site from ADP and inorganic phosphate. Recently, direct observation with a fluorescent probe revealed that the enzyme was a nano-size molecular motor in the membranes. The enzyme shows continuous rotation to one direction by ATP hydrolysis. The proton motive force supposed to rotate the enzyme to the reverse direction for ATP synthesis. Although the rotation is suggested to essential for energy coupling between catalysis and H+ transport, the mechanism was not known. We are studying rotational properties of Escherichia coli mutant enzymes to demonstrate coupling mechanism through rotation.
*Fig. 1 Schematic model of ATP synthase. A subunit complex of γεc10 forms the rotor that rotates against a complex of α3β3δab2, the stator.
2

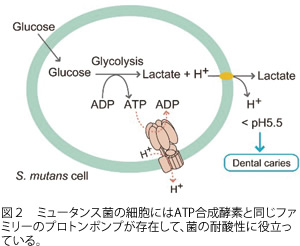
Streptococcus mutans, one of oral microbes, causes dental caries by acidification of tooth surface to secret lactic acid. S. mutans has F-type H+-ATPase in the plasma membrane to maintain cellular pH and survive in acidic environment. The H+-ATPase is a homolog of the ATP synthase, whereas it does not physiologically synthesize ATP by the reverse reaction. Recently, we suggested that the enzyme transported H+ to avoid acidification of cytoplasm. The c subunit has a mechanism to transport H+ at acidic pH, but not at neutral. This subunit would be the drug target site to prevent dental caries.
*Fig. 2 F-type H+-ATPase is located in the plasma membrane of S. mutans. The enzyme actively pumps H+ to maintain cellular pH.
Oka, H., Hosokawa, H., Nakanishi-Matsui, M., Dunn, S. D., Futai, M., and Iwamoto-Kihara, A. Elastic rotation of Escherichia coil F0F1 havingεsubunit fused with Cytochrome b562 or flavodoxin reductase. Biochem. Biophys. Res. Commun. 446, 889-893 (2014)
Sasaki, Y., Nogami, E., Maeda, M., Nakanishi-Matsui, M., and Iwamoto-Kihara, A. A unique F-type H+-ATPase from Streptococcus mutans: An active H+ pump at acidic pH. Biochem. Biophys. Res. Commun., 443,677-682 (2014)
Araki, M., Hoshi, K., Fujiwara, M., Sasaki, Y., Yonezawa, H., Senpuku, H., Iwamoto-Kihara, A., and Maeda, M. Complememtation of FO c subunit of Escherichia coli with that of Streptococcus mutans and the properties of the hybrid FOF1-ATP synthase. J. Bacteriol., 195, 4873-4878 (2013)
Soontharapirakkul, K., Promden, W., Yamada, N., Kageyama, H., Incharoensakdi, A., Iwamoto-Kihara, A., and Takabe, T. Halotolerant cyanobacterium Aphanothece halophytica contains an Na+-dependent F1F0-ATP synthase with a potential role in salt-stress tolerance. J. Biol. Chem. 286, 10169-10176 (2011)
Nakanishi-Matsui, M., Kashiwag, S., Ubukata, T., Iwamoto-Kihara, A., Wada, Y., and Futai, M. Rotational catalysis of Escherichia coli ATP synthase F1 sector. Stochastic f luctuation and a key domain of theβsubunit. J. Biol. Chem. 282, 20698-20704 (2007)






